Describe Experiments to Prepare Insoluble Salts Using Precipitation Reactions
Qualified Chemistry teacher passionate about making engaging activities that help pupils understand and remember key Chemistry concepts. The chemical equation for this precipitation reaction is provided below.

Preparing Insoluble Salts Acid Base And Salt Youtube
The formation of an insoluble salt in water.

. Add enough distilled water to bring the total volume of solution up to the 100 mL mark on the flask or cylinder. Up to 24 cash back needed salt. Save the mixture and the remaining solutions of A and B for later use Describe the appearance of the mixture.
This process is called precipitation. Soluble if it dissolves in water to give a solution with a concentration of at least. Precipitation reactions are used to make insoluble salts.
Soluble and insoluble salts. Use a paper towel to gently soak up any water between the small wells on the surface of the comboplate. The salts made in neutralisation reactions can be either soluble or insoluble.
Edexcel GCSE Science new specification 2011 practical experiment sheet with instructions to fill in while carrying out precipitation reactions. The method of preparing and purifying the salt depends on whether or not it is soluble in water. AgNO3aqueous KCl aqueous AgCl precipitate KNO3aqueous In the above reaction a white precipitate called silver chloride or AgCl is formed which is in the solid.
Up to 24 cash back Add dilute sodium chloride solution to dilute silver nitrate solution until no more precipitate forms. An insoluble precipitate of lead sulphate is formed. Use an empty propette to suck up and then discard any water that may have got into the large wells.
Place a known volume of one soluble salt solution in a beaker. A solution in which a precipitation reaction occurs looks cloudy may be white or colored and if left still the precipitate typically settles to the bottom. The residue is the insoluble salt you are trying to get.
Experiment to prepare insoluble salts using ppt reactions. Mix silver nitrate and sodium chloride together in a beaker Stir the mixture using a glass rod Filter mixture using a filter and filter paper Wash the solid in the filter paper with distilled water because distilled water is. Because of the insolubility of so many lead II compounds the.
I Describe a method to prepare a pure sample of the insoluble salt strontium carbonate by precipitation. Ionic compound containing group 1 metals such as sodium and with nitrate ions are water soluble so NaNO 3is soluble. Choose 2 suitable soluble salts eg.
Because Ag 3PO 4is insoluble we know that there is a precipitation reaction so we go on to Step 3. Up to 24 cash back 48 Describe experiments to prepare insoluble salts using precipitation reactions. I have also produced Biology Physics and Maths resources.
A Complete the table to show which acid or metal compound is used to make each salt listed. Titration must be used if the reactants are soluble. PowerPoint and worksheet for Insoluble Salts.
Insoluble salts can be made by using a precipitation reaction. Precipitation Reactions of Hydrated Ions When examining the hydrolysis reactions of metal cations and oxoanions possible interactions between the cations and anions in solution were ignored. In this episode of Crash Course Chemistry we learn about precipitation precipitates anions cations and how to describe and discuss ionic reactions.
Up to 24 cash back Insoluble salts can be defined as salts that do not dissolve in water at room temperature. Use a propette to add one drop of universal indicator solution into each of the small wells filled with water. Up to 24 cash back 1.
SOME INSOLUBLE LEAD II COMPOUNDS. It describes the reactions to form lead II hydroxide lead II chloride lead II iodide and lead II sulphate. Also sheet to show the steps for making an insoluble salt lead iodide by precipitation reaction.
But when two ions find each other that form an insoluble compound they suddenly fall out of solution in whats called a precipitation reaction. 5 Soluble salts can be made by reacting an acid with a metal hydroxide a metal oxide or a metal carbonate. Add enough distilled water about 20 mL or less to dissolve the salt.
Rubidium carbonate is soluble in water and does not decompose when heated. For each metal compound state whether it would be used as a solid or in. It is insoluble in water and it decomposes when heated.
Precipitation Reaction - Experiment 6 Part I I. Soluble salts can be made by reacting acids with soluble or insoluble reactants. When solutions of two soluble salts are mixed a solid may form.
Dry the insoluble salt crystals. Lead nitrate and sodium sulphate. This page looks at the formation of some insoluble lead II compounds from aqueous lead II ions using precipitation reactions.
During a reaction that uses this method two different aqueous solutions will exchange ions and form two new compounds. Wash residue with water. Filter to collect residue.
Mix two solutions together Filter the mixture Wash with distilled water Then set aside to dry and crystallize. Precipitation reactions with equations. The solid is called a precipitate and the reaction is called a precipitation reaction.
An ionic precipitation reaction occurs when two ionic solutions are mixed and one of the combinations of ions in the new solution creates an insoluble compound which forms a solid in the solution. A lot of ionic compounds dissolve in water dissociating into individual ions. However some cation and anion pairs form insoluble salts.
Make aqueous solutions of both. Insoluble salts can be prepared in the laboratory by using the precipitation method which is also called the double decomposition reaction. Keep adding the other soluble salt solution and stir to mix until no more precipitate forms.
Preparation of insoluble lead sulphate PbSO 4. 11 years of teaching experience. Filter out the precipitate.
In this experiment the soluble salts are magnesium sulfate and sodium carbonate and the insoluble. Insoluble salts are made by precipitation reactions. Wash the residue with cold.
Pour this solution into a 100 mL volumetric flask if available or a 100 mL graduated cylinder. Mix the two aqueous solutions together. Follow me on Twitter for lots of teaching ideas.
Calculate the masses of salts added to each beaker and record the amounts in the data table.

Preparation Of Salts 8 2 1 Cie Igcse Chemistry Revision Notes 2020 Save My Exams

C2 10 Precipitation Reactions Insoluble Salts Teaching Resources
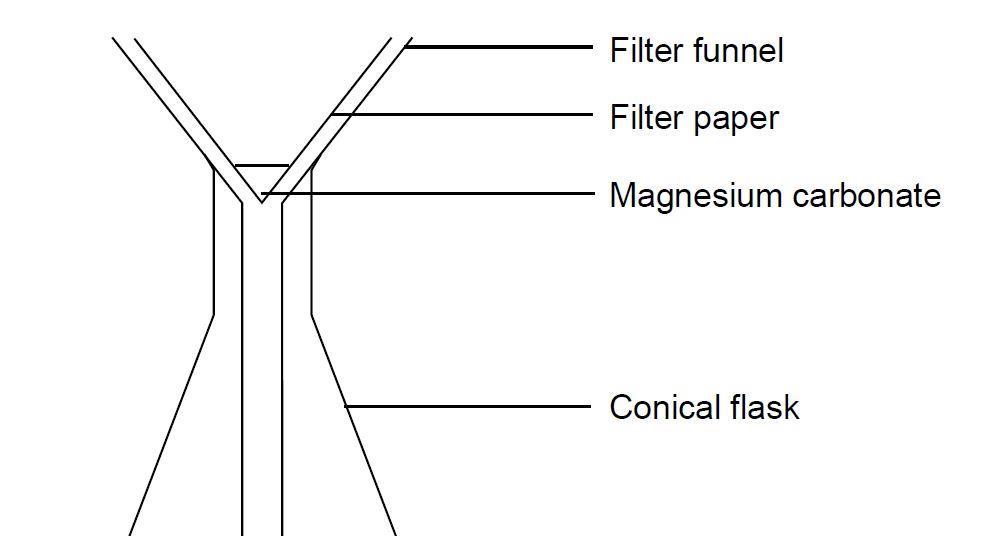
Making Magnesium Carbonate The Formation Of An Insoluble Salt In Water Experiment Rsc Education

How To Make Insoluble Salt Precipitation Method Salt Preparation Online Video O Level Secondary Chemistry Tuition

Acids Bases And Salt Preparations Gcse The Science Hive

Gcse Chemistry Making An Insoluble Salt By Precipitation Youtube

Effect Of Electric Field Experiment 4 Electric Field Electricity Field

Making Insoluble Salts Acids Bases Alkalis Chemistry Fuseschool Youtube

Describing Explaining Methods Of Making Salts Chemical Tests To Identify Salts Ions Gases Compounds Apparatus Chemicals Procedures Equations Chemicals Required Gcse Chemistry Ks3 Ks4 Science Igcse O Level Revision Notes

Procedure Method Making Insoluble Salt By Precipitation Reaction From Two Soluble Compounds Apparatus Chemicals Procedures Equations Use Of Barium Sulfate Meal Gcse Chemistry Ks3 Ks4 Science Igcse O Level Revision Notes

Enthalpy Heat Combustion Experiment 4 Energy Level Heat Exothermic Reaction

Acids Bases And Salt Preparations Gcse The Science Hive

Describe The Preparation Of Soluble And Insoluble Salts Chemistry Lessons Preparation Salt

Total 2 Average 5 5 What Is Meant By Combinational Logic Circuits Combinational Logic Circuits Using Logic Gates Some Logi What Is Meant Logic Circuit
Preparation Of Insoluble Salt Learn Chemistry Corner

Logic Gates Truth Table 1 Logic Gate Electronic Building Blocks
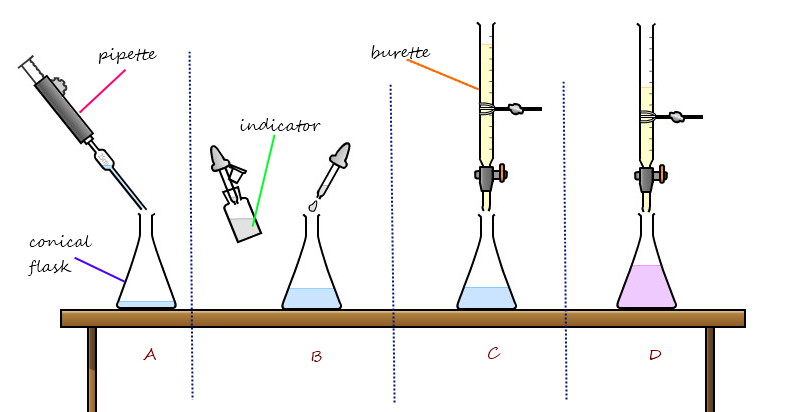
Preparation Of Soluble Salts Insoluble Salts Mini Chemistry Learn Chemistry Online

Making Salts From Solutions Lo Describe What Happens During A Precipitation Reaction D Explain How An Insoluble Salt Can Be Formed C Explain In Detail Ppt Download

Comments
Post a Comment